Semiosis, Evolution, Energy, Development,, Volume 1, Number 1, March 2001
Jack Maze
Department of Botany
University of British Columbia
Vancouver, B. C. V6T 1Z4
Canada
Kathleen. A. Robson
Robson Botanical Consultants
14836 NE 249th Street
Battle Ground, WA 98604
U. S. A.
Satindranath Banerjee
Scientificals Consulting
309-7297 Moffatt Road
Richmond, B. C. V6Y 3E4
Canada
Dedicated to the memory of "Alfie", Alfred B. Acton
This study attempts to address the relationship between ontogeny and phylogeny by analyzing ovules in two different grasses, Achnatherum nelsonii and A. lettermanii, over four different stages in ovule development. The group analyzed at each time period consisted of the ovules of the two species. We evaluated emergence by calculating the degree of emergence seen in each time period. The degree of emergence is the difference in organization, as expressed by correlation matrices, between lower and higher hierarchical levels. Or, the degree of emergence assesses the amount of within-group variation in organization, the result of diverging developmental trajectories. We incorporated time into this study through the different developmental stages that reflect ontogenetic time and the two species that represent phylogenetic time. The degree of emergence increased over the last two developmental stages. Both ontogeny and phylogeny can be viewed as events wherein matter is transformed as energy moves from one state to another, as energy is transformed into information. The expression taken by this information will be determined by the plant in which it occurs as the cytoplasm of the developing organism elicits certain responses from the code stored in the DNA. The changes that occur during ontogeny and phylogeny result from variation in the transformation of energy to information within plants, local scale ontogeny, or among groups of related plants, global level phylogeny.
This paper presents the fifth in a series of empirical studies undertaken to explore the relationship between ontogeny and phylogeny, through exploring emergent properties. Our definitions of ontogeny and phylogeny follow those of Salthe (1993), viz. they are the material processes whereby the observations we label as development and evolution, respectively, come to pass. By material processes we mean those processes that contribute answers to the questions: What changes? How does it change? Why does it change? These questions can be recast, at least in part, as Aristotle's four levels of explanation, sometimes called "causes": "what" the material and formal, his first and second levels of explanation, "how" the efficient, his third, and "why" the final, his fourth.
On a different conceptual level, we wanted to provide a relationship that goes beyond utilizing characters derived from developmental stages in the constructing of phylogenies, or of considering development in terms of the kinds of changes seen as characters evolve, whether in terms of concepts describing relative growth rates (Gould 1977) or the detailed epigenetic events which produce different features (Lovtrup 1974). We want to understand how and why higher level phylogenetic patterns are expressed through local ontogenetic events. Finally, we wanted to anchor this investigation into the conceptual bases of ontogeny and phylogeny in empirical studies.
In a study of the relationship between ontogeny and phylogeny, a first step is to establish a basis for comparison, a feature that will permit use of a common lexicon for the products of ontogeny and phylogeny; for that we elected emergence. One way to view emergence is the biological truism, "the whole is greater than the sum of its parts." That simple statement represents a concept fraught with complexity and difficulty (see e. g., Salthe 1993, Holland 1998). In order to prevent the deflecting of our interests, we chose another definition of emergence, that of Polanyi (1958), viz., higher hierarchical levels, which we take to represent the whole, have properties not seen at lower hierarchical levels, which we take to represent the parts. The properties of the higher level emerge from the properties of the lower but cannot be reduced to, nor fully explained by, the lower level properties. One direct way to characterize Polanyi's formulation of emergence is that descriptions adequate for lower levels (the parts) are inadequate for higher levels (the whole).
Thus we chose to use emergence in our exploration of the relationships between ontogeny and phylogeny. This is accomplished by comparing emergence in the products of ontogeny as seen in individuals, and emergence in the generation of phylogeny, as seen in natural assemblages of individuals, species. From these comparative properties of emergence we hope to infer properties of, and relationships between ontogeny and phylogeny. In order to produce comparable estimates of emergence, it was necessary to use an empirical language that could be applied to both individuals and species. In order to meet this requirement, we used variables that describe both individuals and species and constructed hierarchical levels at both the level of the individual and the species in the same way. This assures us that when we compare emergence between the products of ontogeny and phylogeny our comparisons are not an artifact of using different variables at different hierarchical levels, or differently fabricated hierarchical levels. The former would occur if the between-level differences were one of size, e. g., cells and tissues. Choosing a common set of variables when studying plants is not difficult since these organisms are composed of serial homologues such as leaves or reproductive parts. Furthermore, the existence of serial homologues allows different hierarchical levels to be composed of the same items. For example, if flowers were chosen as sources of variables, the lower hierarchical level would be made up of subsets of flowers, those of each individual, and the higher level would include all of the flowers, of all individuals representing a species.
To date we have explored emergence in individual grasses (Maze and Bohm 1997), in populations, species and a species-pair in grasses (Maze 1998), within individuals of Pinus ponderosa (Maze 1999) and in different aged needle primordia in Pseudotsuga menziesii (Mirb.) Franco (Maze et al. 2000). Of these studies only the last directly addressed a phenomenon pertinent to the present discussion, i. e., ontogeny.
A logical continuation of these studies would be to simultaneously address ontogeny and phylogeny, and to analyze for emergence when their products are combined. We did this by analyzing for emergence in data sets that combined different developmental stages of two species, thus combining ontogeny and phylogeny. The theoretical reason is particularly important; it is through the events of ontogeny that phylogenetic patterns are expressed. Though these patterns are generated through the development of the individual, they cannot be described or explained exclusively at the level of the individual; one must also consider the higher level patterns of speciation in order to discern them.
Time is an integral part of ontogeny and phylogeny. One way to express the relationship between time and these material processes is through Matsuno's (1998) distinctions and relationships between "local" (ontogenetic) and "global" (phylogenetic) time. This distinction leads to a specific question we can address in this study: Are ontogenetic and phylogenetic views of time expressed from the outset or is there an asymmetry in their expression? Is ontogenetic time expressed before phylogenetic, the local before the global? As ontogenetic time is expressed, is phylogenetic time manifested sequentially or from the outset? If ontogenetic time is expressed first, if evolutionary differentiation has a developmental component, we would expect that the degree of emergence would increase as development progresses, as changes related to phylogenetic time appear. Conversely, if the differentiation that has occurred between species is independent of the developmental stages then the degree of emergence should remain unchanged through ontogeny. We attempt to distinguish between these alternatives.
In addition, this study will allow us to test a conclusion from a previous study (Maze 1999): namely that there is a positive relationship between the degree of emergence and variation in organization. As the variation in among-structure (variable) organization increases, there is also an increase in the degree of emergence.
Plants
The plants sampled grew at Tony Lake, 40 km northeast of Logan, Cache Co., Utah (41o 45'N, 111o 30' W) at an elevation of 2560 m. where the two closely related species, Achnatherum nelsonii (Scribn.) Barkworth and A. lettermanii (Vasey) Barkworth, grew sympatrically. Florets were collected at five times in 1984, 29 July, 3, 10, 19 August, and 11 September. At each time, florets from a population were sampled with 10 to 20 plants being represented in each sample. The number of plants per species varied due to the asynchronous flowering times. The florets sampled came from inflorescences that were at least half out of the sheath. This was done to assure that stages preceding megagametophyte development did not predominate in our sample.
The florets collected were preserved in formalin:acetic acid:alcohol (90 cc 50% ethanol:6 cc formalin:4 cc glacial acetic acid). Prior to embedding, ovaries were dissected out of the spikelets, dehydrated with tertiary butyl alcohol, embedded in paraffin, sectioned at 15 micrometers, and stained in safranin and fast green (Johansen 1940). Measurements were made from images reflected onto the projection head of a Zeiss Ultraphot II microscope.
These two species are part of a genus of about 75 species growing mainly in temperate regions of the world (Barkworth 1993). A close relationship between them is beyond doubt but the details of that relationship, as well as to other species in the genus, must wait on more studies within Achnatherum. They were chosen because they grow sympatrically at Tony Grove, simplifying collection and removing the effects of major environmental variation. The species appear to be closely related and we have had a long history of research on the morphology, development relationships and nomenclature of Achnatherum and its relatives starting in 1962 (details on request). As well, Achnatherum and its relatives form a large natural group, the grasses. Grasses are abundant, diverse and easy to study, thus allowing us to expand studies on emergence by adding more and more species of varying degrees of relationship. The age of these species is unknown although related genera have been reported from the late Tertiary of the high plains of Kansas (Elias 1942).
Data
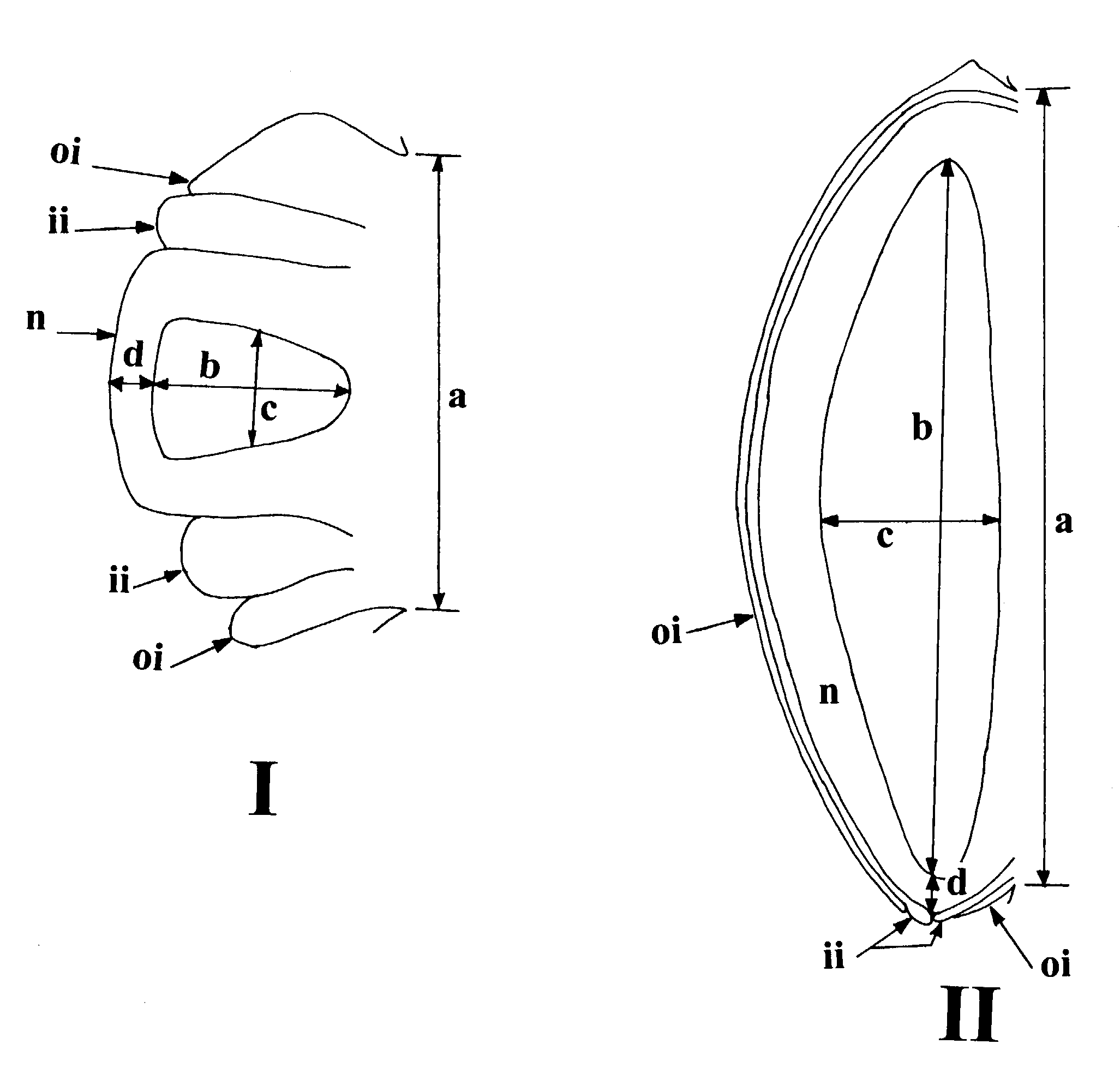
Variables measured were taken from sagittal sections of ovules. Those chosen were designed to describe the various linear distances within the ovule. Due to the bending that occurs as grass ovules develop, some caution had to be exercised to assure that comparable features were being measured at different stages of development. The only features that met the criterion of among stage comparability, and those chosen for analysis, were OV1 (a), length of the ovule attachment at the placenta) OV2 (b), length of the archesporium), OV3 (c), width of the archesporium at its midpoint), OV4 (d), distance from the archesporium to the distal portion of the ovule). The features are shown in Figure 1.

Figure 1. Measurements made on ovules, early stage (I) and late (II). a, OV1 (length of the ovule attachment at the placenta); b, OV2 (length of the archesporium); c, OV3 (width of the archesporium at its midpoint); d, OV4 (distance from the archesporium to the distal portion of the ovule). ii, inner integument; oi, outer integument; n, nucellus.
The first step in analysis was to determine developmental stages to use in making comparisons. Because the analytical method used relied on principal components analysis (PCA) of a correlation matrix we had to assure that each stage was of sufficient sample size (n>26, Pimentel 1993) to give us confidence in the results of the PCA. An initial plan to use date of collection was abandoned because of the tremendous variability within each collecting date; the stages represented in the ovules collected on any one date ranged from a low of three (11 Sept.) to a high of eleven (3 Aug). Thus we used stages of development of the archesporium as a means to establish developmental stage, the stages being: ovules having megaspore mother cells being assigned to stage 1, diads 2, tetrads 3, megaspores 4, two nucleate megagametophytes 5, four nucleate megagametophytes 6, eight nucleate megagametophytes 7, mature megagametophytes 8, zygotes and few endosperm nuclei 9, two celled embryos 10, and four celled embryos 11. These groupings still did not give sample sizes adequate for the desired analyses, so we grouped ovules in different stages of development. Thus, analytical stage 1 included ovules at stages 1 - 5, analytical stage 2 consisted of ovules at stages 6 and 7, analytical stage 3 of ovules at stage 8 and stage 4 was comprised of ovules at stages 9 - 11.
In spite of combining stages, we can make valid comparisons. Included in Figure 2 are representative ovules for stages 1 - 4. The range of form within any one of those
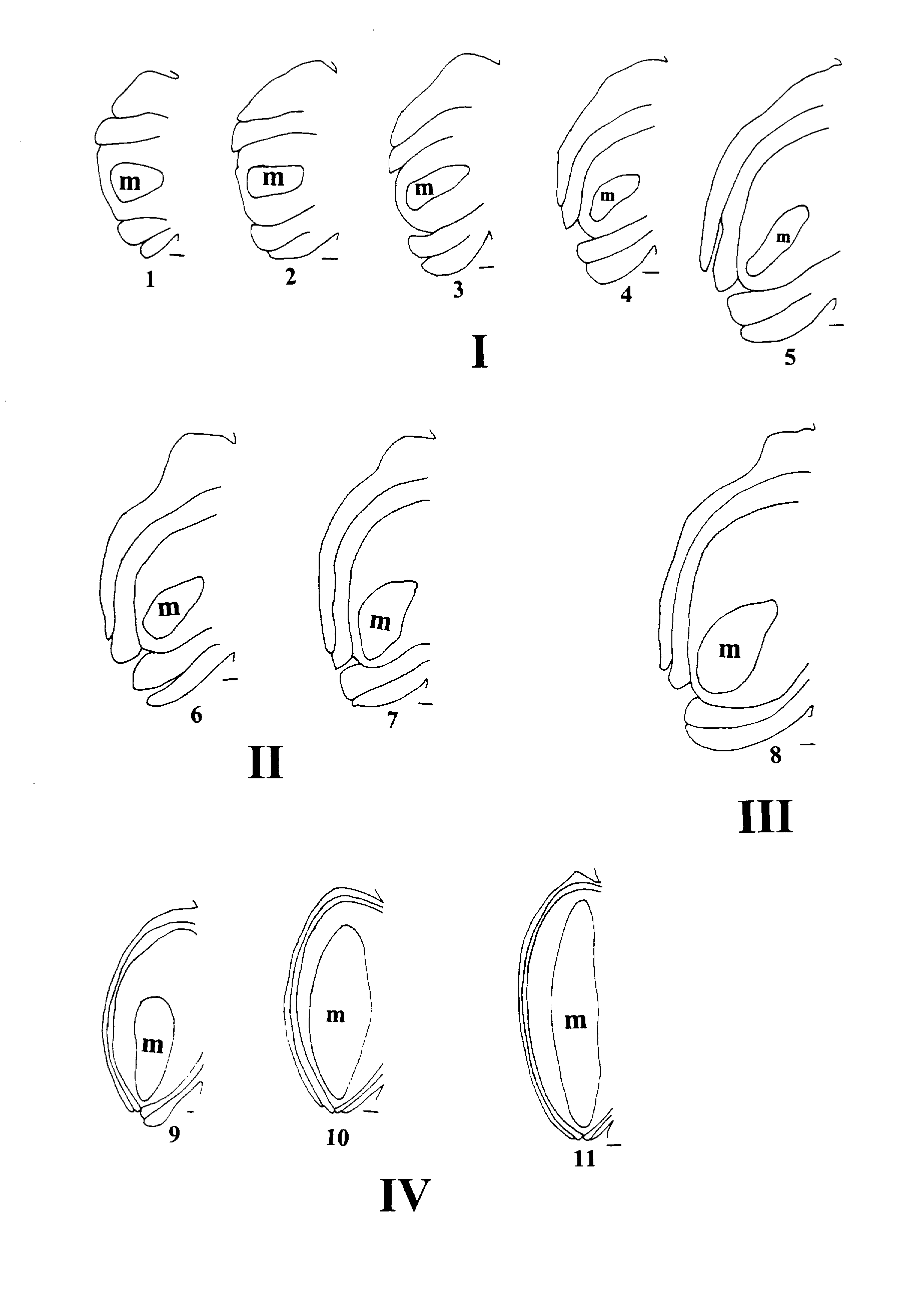
Figure 2. Diagrams representing 11 stages of development in Table 1 showing the stages combined into the analytical stages. Analysis stage 1, stages 1-5 (I); analysis stage 2, stages 6 and 7 II); analysis stage 3, stage 8 (III); analysis stage 4, stages 9 - 11 (IV); m, archesporium. Lines by each diagram indicated scale, for stages 1 - 9, 0.01 mm; for stages 10 and 11, 0.1 mm.
synthetic groups is not extreme, aside perhaps from some possible ovules in stage 4. In order to prevent undue variation of ovules in stage 4 having an effect on our results, we excluded outliers based on OV2, the length of the archesporium.
The variables used in this study are independent of those that differentiate A. nelsonii and A. lettermanii. The two species are similar although the former has larger individuals with wider leaves, larger inflorescences and larger spikelets. The latter species has a tuft of obviously longer hairs at the apex of the floret and a longer palea. There has never been an embryological comparison of these two species. Thus any results from this study will not be the end result of relying on features of known systematic significance.
Analyses
The general approach was the same as that of Maze and Bohm (1997), Maze (1998), Maze (1999) and Maze et al.(2000). Groups were analyzed for the degree of emergence based on a comparison of angles formed between eigenvectors from a PCA and a vector of isometry. The specific comparison made was between those angles for subgroups, a lower hierarchical level, and the same angle for the sample, which was comprised of those subgroups combined into one, a higher hierarchical level. The difference between those angles, called the average degree of emergence (AVGD), was the statistic used to evaluate emergence, the greater the AVGD the greater the emergence.
We wanted to combine ontogeny and phylogeny in one analysis. The most reasonable way to do that seemed to be a comparison of the two species for the four stages analyzed, ontogeny is captured in the four developmental stages, phylogeny in the two species. Thus we calculated AVGD for the two species for the four stages analyzed: For each analysis one subgroup consisted of A. lettermanii and the other of A. nelsonii, and AVGD would be the average degree of emergence shown for both species when they are combined together in one analysis.
As in the previous studies on emergence (Maze and Bohm 1997; Maze 1998, 1999) the data were bootstrapped (Efron 1982). Random samples from each group analyzed were generated using SYSTAT 4.1 (Wilkinson 1988) which uses an algorithm developed by Bebbington (1975). The PCAs were done using Pimentel's MPCA (1993) program. Pimentel's (1993) program is well suited to a study such as this since the statistics for the entire data set, the within-groups analysis, is derived from the within-groups dispersion matrix, the weighted average of the group dispersions (Pimentel 1993). This approach is preferred since it is less sensitive to assumptions about the within-group dispersions, such as equality, that describe the lower hierarchical levels. For details see Pimentel (1979).
The comparison in AVGD, our estimator of emergence, for the different stages was done using a non-parametric technique, Kruskall-Wallace analysis of variance with the probability of rejection of a hypothesis of similarity set at p << 0.001. This conservative level was taken and a non-parametric statistic used because of the high degree of manipulation involved in these analyses.
A third analysis was done, a comparison of AVGD with two variables, variation in size and what we call variation in shape. The first is estimated by within stage variation in first PCA axis scores for a PCA on all four stages combined. The second is approximated by the variance in the first eigenvector loadings for within-stage PCA for each stage separately. The logic behind using eigenvector loadings as an estimator of what we call shape is the relationship between shape and organization and the relationship between organization, as revealed in a correlation matrix, and the first eigenvector derived from such a matrix (Maze et al.1987).
A final analysis was a comparison of angles between eigenvectors for the two species for each stage of development. This comparison, like that of the PCAs was done using Pimentel's (1993) program.
In all the comparisons made only the first PCA axis was used because it accounts for the vast majority of the variation in the data, 64.262% averaged over all analyses. As well, it was only the first axis that was consistently derived from a non-spherical data set as indicated by Bartlett's test (Pimentel 1993).
Our analyses appear to be very complex; we suspect that complexity is mainly the result of many manipulations rather than innate complexity. There is a way to visualize our approach that may help to clarify our methods. This visualization relies on the relationship between the scatter gram from a PCA and the correlation matrix from which that PCA is derived. A certain correlation matrix will produce a two-dimensional scatter diagram, often ellipsoidal in shape and with a certain orientation, from a PCA. A different correlation matrix will produce an ellipse with a different shape and orientation. To put that in the context of our use of emergence, subgroups which represent the lower hierarchical level will have ellipses of different shape and orientation than will an ellipse from the subgroups combined, the higher hierarchical level. Furthermore, the size, shape and orientation of ellipses will change over developmental time. This is represented in Figure 3
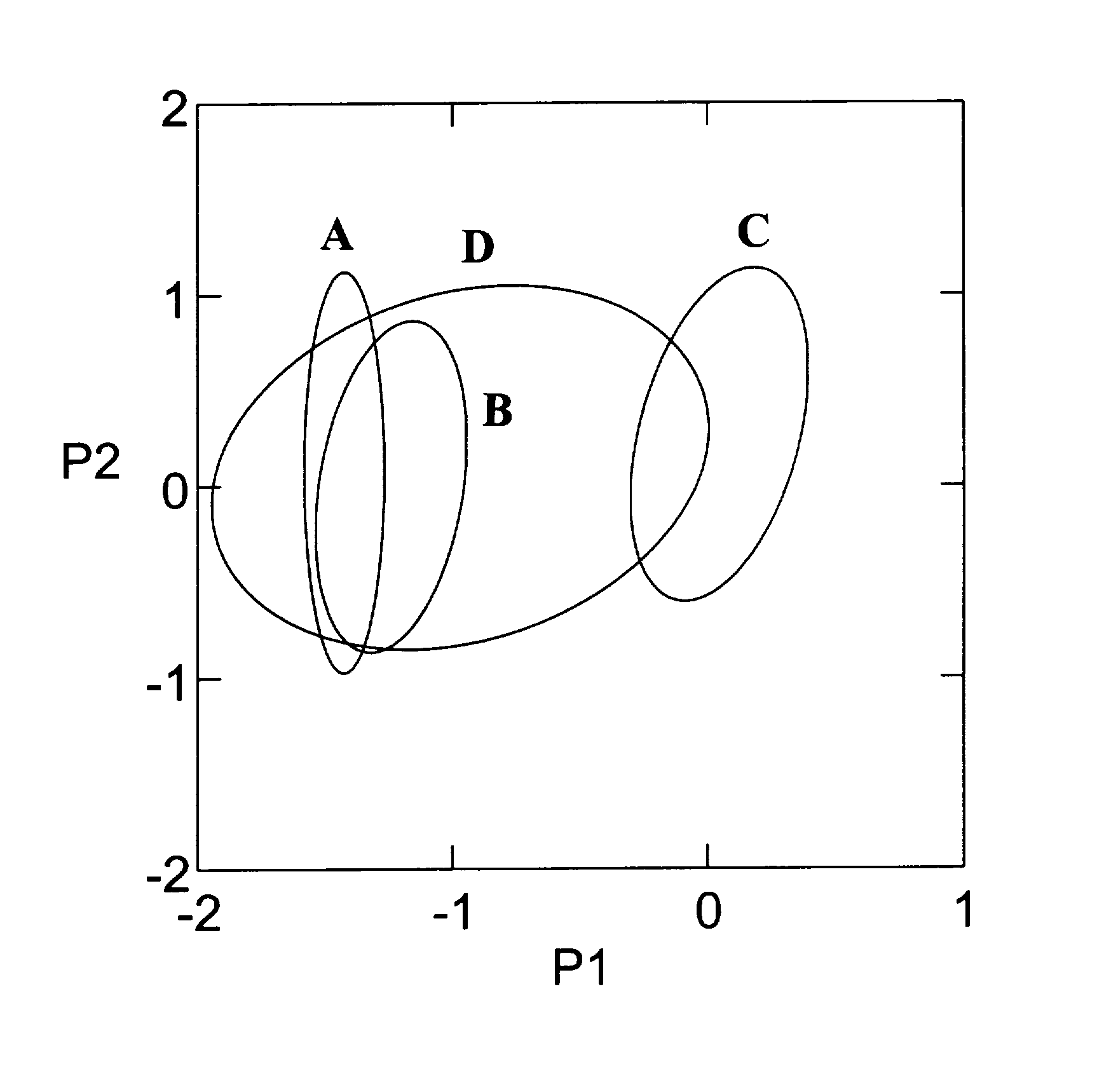
Figure 3. Diagram showing ellipses derived from subgroups (A, B, C) and from the subgroups combined (D). P1, first PCA axis; P2, second PCA axis.
where three ellipses labeled A, B and C are derived from subgroups, the lower level and ellipse D, with its different orientation, represents all groups combined, the higher level.
That visualization relates to our analyses through the relationship between the angle with a vector of isometry and a correlation matrix, different correlation matrices will produce different angles with a vector of isometry. It is these angles that we compare. The bootstrapping is required in order to make a meaningful comparison among only three numbers, angles for the subgroups (two of the numbers) and an angle for the entire group (the third number). By bootstrapping the data these three numbers are represented as means which can then be compared.
Achnatherum nelsonii is the first species to develop as indicated by the earlier appearance of any one stage in ovule development. It also has ovule development occurring over the longest period of time since it alone still showed developing ovules on 11 Sept. and has the greatest number of ovules on all collecting dates aside from August 19. The predominant stage is the mature megagametophyte, that within which cell walls have formed, followed by the pre-cytokinesis eight-nucleate megagametophyte.
There is an increase in the degree of emergence (AVGD) for the two species over the course of time, time being estimated by the developmental stages established (Table 1).
Table 1. Average degree of emergence (AVGD), as median values, for stages 1 - 4 for the two species. Lines connecting stages mean medians are the same at p < 0.000000
Stage 1 2 3 4
AVGD 1.00 0.86 3.06 9.15
____________________ ____ ____
Stages 1 and 2 are the same, there is a significant increase in AVGD for stage 3 and another for stage 4. And there is a greater effect of variation in shape on AVGD than variation in size (Table 2).
Table 2. Results of multiple regression analysis with the average degree of emergence, AVGD, as the dependent variable. SIZEVAR, variation of ovules as measured by variance in PCA axis scores for stages 1 - 4; SHAPEVAR, variation in integration for each tree as measured by variance in loadings on the first eigenvector.
r2 = .569
variable coefficient prob
SIZEVAR 0.565 0.047
SHAPEVAR 2.526 0.000
Table 3 presents, for the four stages of development, the first eigenvectors for each species and the angles between those eigenvectors.
|
A. nelsonii |
A. lettermanii |
|
| Stage 1 OV1 OV2 OV3 OV4 ANG 6.9 |
0.860 0.752 0.859 0.703 |
0.920 0.920 0.764 0.829 |
| Stage 2 OV1 OV2 0 OV3 OV4 ANG 6.0 |
0.854 0.854 0.764 0.571 |
0.857 0.915 0.699 0.726 |
| Stage 3 OV1 OV2 OV3 OV4 ANG 10.6 |
0.932 0.936 0.837 0.505 |
0.916 0.926 0.711 0.788 |
| Stage 4 OV1 OV2 OV3 OV4 ANG 17.8 |
0.791 0.908 0.804 -0.464 |
0.918 0.883 0.786 -0.014 |
Table 3. First eigenvectors for the four stages of development for each species and angle between the first eigenvectors. OV1, length of the ovule attachment at the placenta; OV2, length of the archesporium; OV3 width of the archesporium at its midpoint; OV4 distance from the archesporium to the distal portion of the ovule; ANG, angle between eigenvectors.
The main point of interest is the increasing angle between the eigenvectors, especially at stage 4 This increase in angle between eigenvectors can be linked to OV4 (distance from the archesporium to the distal portion of the ovule); it declines in both species but more so in A. nelsonii.
In previous studies there was a strong relationship between the degree of emergence (AVGD) and variation in shape (Maze and Bohm 1997; Maze 1998, 1999; Maze et al. 2000); the same is seen here. Due to a relationship between the concepts of shape and organization (Maze et al. 1987) what we are seeing is a relationship between emergence and variation in organization. Furthermore, variation in organization is the result of variation in growth rates of the parts relative to each other. In this study the variation in growth rates increases with time.
Another way to envision the increase in variation in growth rates is as diverging developmental trajectories. With time the trajectories occupied by the ovules of A. nelsonii and A. lettermanii diverge from each other as is seen in the increasing angles between first eigenvectors (Table 3). Each ovule itself consists of developmental trajectories seen in the length of the ovule attachment to the placenta (OV1), the length of the archesporium (OV2), the width of the archesporium (OV3) and the distance from the archesporium to the distal portion of the ovule (OV4). It is in OV4 though where there is the greatest divergence in developmental trajectory. Salthe states, "In fact, trajectories are the cohesion of systems (here OV1, OV2, OV3, OV4), and 'their twisting' together represents the cohesion of still-higher-scalar-level entities" (1993: 182). That account emphasizes that the trajectories of OV1, OV2, OV3 and OV4 are cohesive because of properties of the ovules. And a similar interpretation can be applied to even higher levels; the ovules of these grasses have cohesiveness imposed on their trajectories by the florets and the inflorescence. And so it goes, higher and higher up the scalar level of the biological hierarchy until the level of the individual plant is reached. And it is the individual plant that offers cohesiveness for the plant. The imposition of cohesiveness on developmental trajectories by an individual offers a mechanism consistent with Hofmeister's (1867) argument (Kaplan 1992) that it is the individual plant that determines the nature of the parts. At other levels of organization there is also the historical cohesion that is typical of Achnatherum florets and inflorescences, of grasses, and of flowering plants.
Since the degree of emergence is calculated for each developmental stage analyzed, the variation being assessed is within-stage, between-species variation. Because the degree of emergence increases for each stage of development and because of a relationship between emergence and variation, it is tempting to speculate, as in Maze (1999), that there is some sort of causal relationship between variation and emergence. And because emergence is a form of differentiation (Maze 1998, 1999) it may be argued that a causal relationship exists between variation and differentiation where variation, constrained during growth and development by the organisms in which it occurs, leads to emergence.
An expanded account of this combination of constraint and variation can be found in Taborsky’s (1998) perspectives on semiotics. As energy flows through a plant the matter that constitutes the body of the plant is transformed from one state to another; as energy is passed along an energy gradient, information is constructed out of matter (Taborsky 1999). The specific way that matter is transformed is determined by the plant within which that transformation occurs, this being determined by the reaction the cytoplasm elicits from the DNA. This transformation of matter can be, at the simplest, the synthesis of cellulose out of glucose residues or, at the more complex, the formation of a set of cell walls with a certain orientation in a certain position to form ovules and florets typical of Achnatherum. The differences in the ways that matter is transformed in A. nelsonii and A. lettermanii is seen in the diverging developmental trajectories related to the different growth rates of OV4.
The emergence we have described here can also be characterized as the result of "symmetry, universalization or generalization" for constraint and, "asymmetry, particularization, specification" for variation. "We know them scientifically as the laws of conservation of energy and the entropic dissipation of energy." or as inertia and thermodynamics (Taborsky 1999). In terms of time, constraint takes place in globally synchronous time while variation occurs in locally asynchronous time (Matsuno 1998). Thus, in the plants being studied here, the increase in emergence that occurs with development is the result of the progression of ontogenetic events in locally asynchronous time that simultaneously access and express phylogenetic information in the globally synchronous time from which is derived constraint and inertia. This continual addition of cellular communications and change in locally asynchronous time and the manifestation of global, species-level properties results in diverging developmental trajectories seen in the ovules for the two species. To put these ideas in still another context, constraint, symmetry, conservation and inertia find expression as Polanyi's (1976) boundary conditions. It is the boundary conditions that determine the path of energy transfer and the specific transformations of matter that accompany that transfer. As well, with either development or evolution, there will be a feedback between transformation and constraint with each transformed state forming constraint for subsequent steps. As growth occurs information is generated by local interactions and also accessed from the global historical record (Matsuno 1998). The resulting organisms express an increase in both individual novelty and historical (phylogenetic) predictability as they mature.
Invoking energetic concepts as alternate expressions for constraint and variation places them in a context that extends from biology alone to the more general realm of thermodynamics. A recognition of the consistency between the laws of thermodynamics and organismic evolution and development where the dissipation of entropy is expressed as partly unpredictable, but organized and functional, local novelty that is limited by phylogenetic history can be traced to (Brooks and Wiley 1986). Several research programs have developed around the ideas introduced there (Banerjee et al. 1990; Brooks 1997, 1998; Brooks and McLennan 1999; Brulisauer et al. 1996; Honda et al. 1995; Matsuno 1997; Salthe 1999; Ulanowicz 1997). As energy moves along energy gradients, matter is transformed from one state to another; energy is transformed into information through the process of semiotics (Taborsky 1999) and the generation of local time through cellular communication. Relating biological change to the transformation of energy also introduces inevitability to biological change; given the existence of life it is inevitable that change will occur. This idea of the inevitability of change is not a new one in biology. It first appeared as Lamarck's "power of life," the inevitable drive towards increasing complexity that characterizes life (Lamarck 1803).
In the introduction we stated this problem in terms of Aristotle's four levels of explanation, material, formal, efficient and final. We feel we can now place details derived from this study within that Aristotelian framework. The material and formal, which can't really be separated, would be the cellular components of the ovules of A. nelsonii and A. lettermanii and how they are organized into ovules. The efficient would be the transformation of matter that occurs as energy flows into the ovules. The final is the inevitability of the flow of energy (Taborsky, 1998).
Banerjee, S., P.R. Sibbald and J. Maze: 1990, Quantifying the dynamics of order and organization in biological systems, J. Theor. Biol. 143:91-111.
Barkworth, M.E.: 1993, Achnatherum, The Jepson Manual, James C. Hickman (ed.) Univ. California Press, Berkeley, CA.
Bebbington, A.C.: 1975, A simple method of drawing a sample without replacement, Applied Statistics 24:136.
Brooks, D.R.: 1997, Biological evolution as a microcosm of cosmological evolution, Bridges 4:9-35.
Brooks, D.R.: 1998, The unified theory of evolution and selection processes, Evolutionary Systems: Biological and epistemological perspectives on selection and self-organization, G. van de Vijver, S.N. Salthe and M. Delpos, (eds.), Kluwer Academic Publishers, Dordrecht.
Brooks, D.R. and D.A. McLennan: 1999, The nature of the organism and the emergence of selection processes and biological signals, Semiosis.Evolution.Energy, E. Taborsky (ed.), Shaker Verlag, Aachen, pp. 185-218.
Brooks, D.R. and E.O. Wiley: 1986, Evolution as entropy, 1st ed., University of Chicago Press, Chicago.
Brulisauer, A.R., G.E. Bradfield and J. Maze: 1996, Quantifying organizational change after fire in lodgepole pine forest understorey, Can. J. Bot. 74:1773-1782.
Efron, B.: 1982, The jackknife, the bootstrap and other resampling plans, SIAM Monograph number 38, Society for Industrial and Applied Mathematics, Philadelphia.
Elias, M.K.: 1942, Tertiary prairie grasses and other herbs from the high plains, Geol. Soc. Amer. Spec. Papers 41:1-176.
Gould, S.J.: 1977, Ontogeny and phylogeny, Harvard University Press, Cambridge
Hofmeister, W.: 1867, Die Lehre von der Pflanzenzelle: Handbuch der Pflanzenphysiologie, Vol. 1, pt. 1, Engelmann, Leipzig.
Holland, J.H.: 1998, Emergence. From chaos to order, Addison Wesley.
Honda, H., S. Nagao, K. Hatori and K. Matsuno: 1995, Slow and macroscopic modulation of thermal fluctuations in myofibrils, Biophys, Chem. 54:61-66.
Johansen, D.A.: 1940, Plant microtechnique, McGraw-Hill, New York.
Kaplan, D.R.: 1992, The relationship of cells to organisms in plants: Problems and implications of an organismal perspective, Int. J. Plt. Sci. 153(Suppl):S28-S37.
Lamarck, J.B.: 1803, Zoological philosophy, Translated by H. Eliot (1984), University of Chicago Press, Chicago.
Lovtrup, S.: 1974, Epigenetics: a treatise on theoretical biology, Wiley - Interscience, New York.
Matsuno, K.: 1997, Biodynamics for the emergence of energy consumers, BioSystems, 42:119-127.
Matsuno, K.: 1998, Dynamics of time and information in dynamic time, BioSystems, 46,57-71.
Maze, J.: 1998, Studies into abstract properties of individuals. II. Analysis for emergence in populations, species and a species-pair, Int. J. Plt. Sci. 159:687-694.
Maze, J.: 1999, Studies into abstract properties of individuals. III. A study of factors affecting emergence, Int. J. Plt. Sci. 160:809-817.
Maze, J. and L.R. Bohm: 1997, Studies into abstract properties of individuals. I. Emergence in grass inflorescences, Int. J. Plt. Sci. 158:685-692.
Maze, J., K.A. Robson and S. Banerjee: 2000, Studies into abstract properties of individuals. IV. Emergence in different aged needle primordia of Douglas fir, BioSystems 56:43-53.
Maze, J., R. Scagel and L.R. Bohm: 1987, Quantitative studies in ovule development. III. An estimate of shape change in Phyllostachys aurea, Can. J. Bot. 65:1531-1538.
Pimentel, R.A.: 1979, Morphometrics, the multivariate analysis of biological data, Kendall/Hunt Publishing Co, Dubuque.
Pimentel, R.A.: 1993, BIOSTAT II: A multivariate statistical toolbox, Sigma Soft, San Luis Obispo.
Polanyi, M.: 1958, Personal knowledge, University of Chicago Press, Chicago.
Polanyi, M.: 1976, Life's irreducible structure, Topics in the philosophy of biology, Boston studies in the philosophy of science, M. Grene, E. Mendelsohn (eds.) (D. Reidel Publishing Co., Boston), pp. 128-142.
Salthe, S.N.: 1993, Development and evolution: complexity and change in biology, MIT Press, Cambridge.
Salthe, S.N.: 1999, Energy, development, and semiosis, Semiosis.Evolution.Energy, E. Taborsky (ed.), Shaker Verlag, Aachen, pp. 245-261.
Taborsky, E.: 1998, Architectonics of semiosis, St. Martin's Press, New York.
Taborsky, E.: 1999, Evolution of consciousness, BioSystems, 51:153-168.
Ulanowicz, R.E.: 1997, Ecology, the ascendant perspective, Columbia University Press, New York.
Wilkinson L.: 1988, SYSTAT: The system for statistics, SYSTAT Inc, Evanston.